Photobiomodulation
What is Photobiomodulation?
Photobiomodulation (PBM) is the metabolic and cytological response of living cells to photons of “light energy”.
Photobiomodulation (PBM) is the metabolic and cytological response of living cells to photons, i.e. light energy, comprising electromagnetic radiation (EMR) in the visible spectrum and in portions of the near infrared (NIR) and ultraviolet (UV) bands. [1]
A concatenation of “photo” meaning light, “bio” meaning “living cells”, and “modulation” to vary or exert an influence on, the term photobiomodulation describes biochemical reactions that occur in living cells in response to light [2].




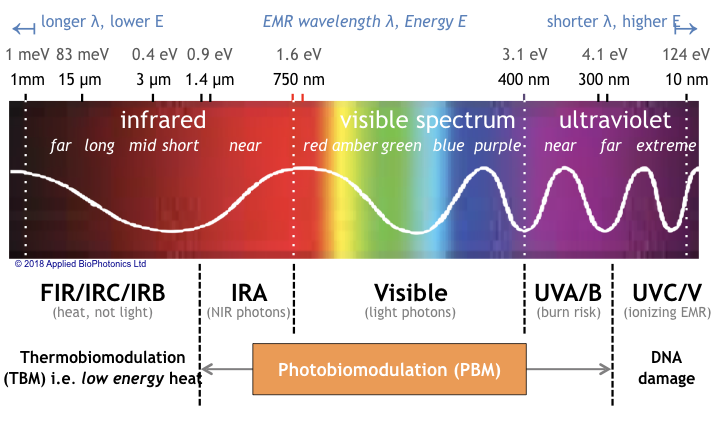
Although electromagnetic radiation interacts affects living creatures throughout the entire spectrum, photobiomodulation is limited to only certain spectral bands. Specifically, as shown in the following figure, PBM occurs in the NIR, visible, and ultraviolet bands of the electromagnetic spectrum (light having wavelengths from 1.4 µm to 300 nm) bounded between long wave portions of the infrared spectrum referred to as IRB (generating only heat) and short wave UVC comprising ionizing radiation (which damages DNA).
Note that in this portion of the electromagnetic spectrum [5], it is customary to describe colors by the light’s wavelength [6] rather than is frequency (light frequencies are inconveniently large to represent numerically). Likewise energy is described in electron-volts (eV) [7], not in Joules (because the energy of a single molecule is too small to conveniently represent in Joules).
Since photons in the NIR and visible band carry two orders-of-magnitude more energy (> 1 eV) than far infrared photons (10–3 eV), the action mechanism of PBM is primarily chemical, not thermal. In contrast, long-wave IRB and IRC photons generate heat (photo-thermal effects) but contain insufficient energy to directly invoke photochemical or photobiological mechanisms.
As such PBM is profoundly different in its action mechanisms from that of heat therapy, i.e. “thermobiomodulation” (TBM) – treatments delivered by infrared saunas, heating pads, steam rooms, and jacuzzis. Because of its energy advantage, light therapy generally outperforms heat therapeutically.
Photobiomodulation occurs naturally in the presence of sunlight and also in artificial light. The impact of light on living cells may be beneficial or injurious, depending on the photonic energy absorbed, parametrically described by the light’s
- Wavelength aka color (µm or nm)
- Power density aka irradiance (W or W/cm2)
- Total energy (dose) aka fluence, in (eV, J, or J/cm2)
Responses vary by organism, tissue and cell type. Wide spectrum light sources (like sunlight) typically include both helpful and harmful rays, the net impact of which depends on light’s color temperature, i.e. spectral blend, and on the total energy dose at each constituent wavelength.
Living organisms are easily damaged by shortwave ultraviolet light UVC with its high energy content (E > 4 eV). Fortunately natural UVC and short-wavelength EMR emitted from the sun (such as X-rays & gamma rays) are blocked by the earth’s magnetic field, ionosphere, and by its atmosphere [8] (including stratospheric ozone (O3) and water vapor in the troposphere). Without this protective shroud, constant DNA damage would threaten the sustainability of life itself.
Because of the risks of skin damage, burns and skin cancer, doctors’ therapeutic use of artificial ultraviolet light sources (i.e. λ < 400 nm) is avoided (except to treat severe skin conditions unresponsive to all other therapies). Ironically the non-medical use of UV light in tanning salons does not fall under FDA jurisdiction, essentially operating as a unregulated medical industry [9]
In contrast, the medical application of PBM as a therapeutic regimen (described later) is subject to strict medical device regulation (typically as a Class II device or possibly as Class III/IV lasers). Treatments are generally performed within a well established safe range of wavelengths occupying the near infrared (NIR, IRA) and visible light spectrum between 950 nm and 400 nm.
Sources of light for therapeutic PBM vary by manufacturer and by design. Aside from the unpopular (and nearly obsolete) methods of using filtered sunlight (uncomfortable) and gas discharge tubes (breakable), most therapeutic PBM today employ electronically controlled artificial light sources comprising lasers or arrays of LEDs designed to emit precisely-specified bandwidths of EMR. Although laser PBM operates at low power densities (cold lasers), the risk of malfunction still represents an eye safety risk [10], requiring the supervised use of safety glasses. Intrinsically, LED based systems do not present a blindness risk because at high currents the LEDs get warm and lose quantum efficiency, limiting their brightness to within safe levels.




Plants Aren’t the Only Source of Biotic Energy
Throughout the 20th century, biologists, botanists, and educators confidently purported that all life on earth obtains its energy from sunlight stimulating photosynthesis in plants. In the photosynthetic process, chloroplasts located in plant leafs convert sunlight (photonic energy) and raw materials (hydrogen, oxygen, carbon) into simple sugars (glucose), storing energy in the plants in the form of carbohydrates [11]. Animals consuming this vegetation ingest these carbohydrates, converting them into energy (ATP) and storing fat for fueling metabolism.
Dying plants and animals in turn provide a food source for bacteria involved in the processing of decay & decomposition, recycling raw elements for reuse in successor life cycles. This closed biotic loop forms the well publicized food chain (see figure) of our earthly biosphere [12].
Not all dead creatures however, decompose into simple elements and low-molecular weight byproducts. In the “biogenic theory” the death of PBM-powered plants and animals followed by bacterial action, sedimentation and hundreds-of-thousands of years of heat and pressure, are thought to transform a portion of these biological corpses into dark, waxy organic layers known as “kerogen” [13]. Kerogen is believed to be one of several precursors of petroleum oil, the high-molecular weight organic compounds storing vast quantities of energy (hence the term “fossil” fuel). Given the stability of crude oil molecules, the raw materials of decaying organic matter once converted into petroleum are permanently removed from the food chain.
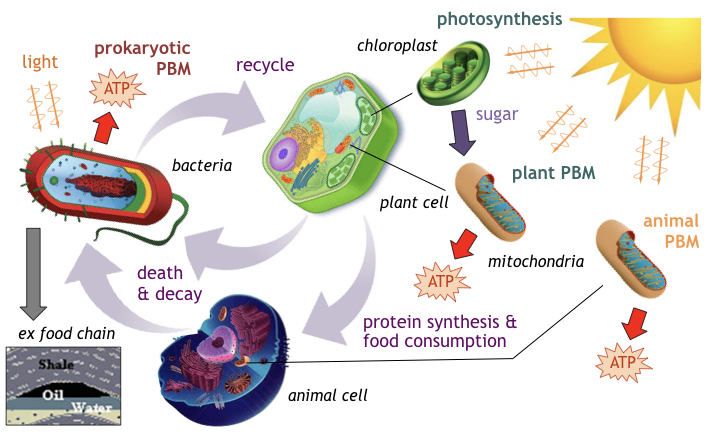
- Photosynthesis in chloroplasts is not the only method to convert sunlight into energy. Bacteria, archaea, and animals also possess the machinery and photochemical mechanisms able to absorb light and directly convert it into usable and stored energy [14].
- PBM’s direct conversion process employs light absorbing chromophores typically located in the membranes of cells and organelles. For example, the organelle mitochondria present in both plant and animals is able to convert sunlight directly into ATP [15] without relying on chloroplast-manufactured sugars (i.e. PBM without photosynthesis).
- Some bacteria are able to produce their own energy either by absorbing light to generate ATP, by fixating sulfur compounds (present near volcanic vents) [16], i.e. producing ATP without any light whatsoever, or some combination thereof.
- Theoretical evidence suggest organelles present in eukaryotic cells likely existed previously as free floating prokaryotes [17], later absorbed into host eukaryotes for symbiotic survival advantages (the endosymbiotic theory).
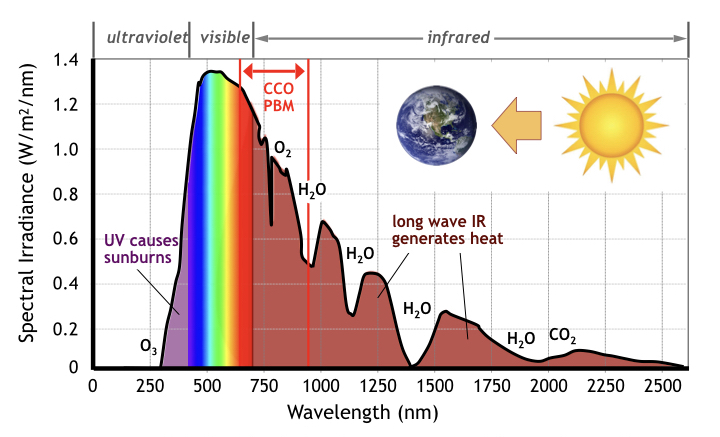
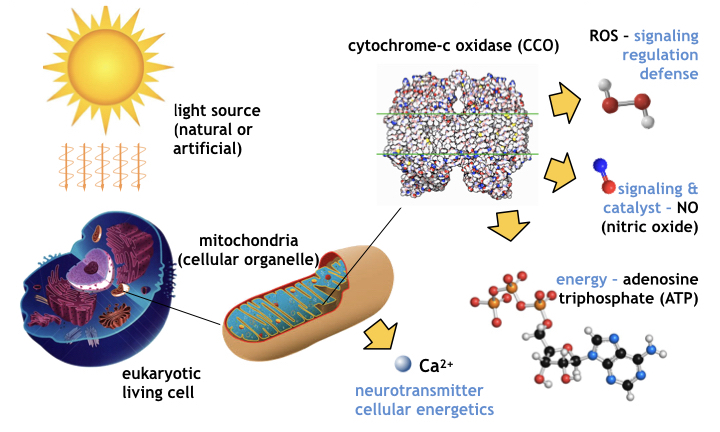
Animal PBM occurs primarily from optical absorption of chromophores in the molecule cytochrome-c oxidase (CCO) associated with the action of mitochondria (click on PBM tab above for more detail). CCO photobiomodulation occurs in a transparent optical window [18] of wavelengths in the band from red light (650 nm) to near infrared light (950 nm) where light is not significantly absorbed by either water (H2O) or by blood, i.e. oxygenated and de-oxygenated hemoglobin (labeled OxyHb and DeoxyHb respectively). [Click on PBM tab for more details]
In photobiomodulation, light must be absorbed to invoke a photochemical, photobiological, or physiological response. But absorption alone is not enough to ensure a PBM response. Instead PBM activity is described by its “action spectra”. As described by Hartmann in 1983, “An action spectrum [19] is a plot of the relative effectiveness of different wavelengths of light in causing a particular biological response, and under ideal conditions, it should mimic the absorption spectrum of the molecule that is absorbing the light, and whose photochemical alteration causes the effect.”
And although the action spectrum of copper subgroups have been confirmed as distinct manifestations of cytochrome, the optical absorption spectrum of CCO is not the same as its action spectra. The action spectra of CCO was studied extensively by Karu et al. using mammalian cells stimulated with varying wavelengths of light in the visible and near infrared spectrum [20] (see following figure). Other studies have identified variants of action spectra [21].
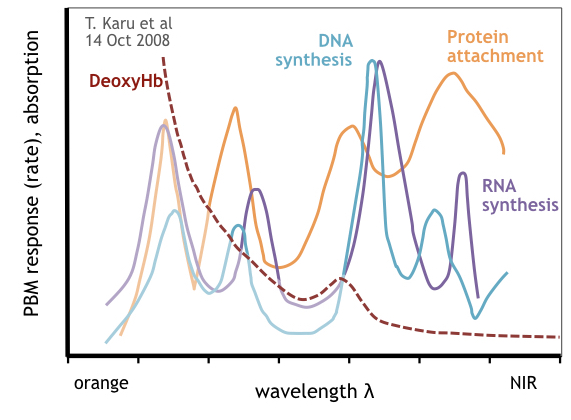
Measuring the rates for DNA synthesis, RNA synthesis, protein generation and attachment (RNA transcription) over a rage of wavelengths, several observations were made:
CCO action spectra has four distinct peaks different than the CCO optical absorption spectra which exhibits only two major peaks (plus one smaller secondary peak).
The peaks and maximum rate points for DNA synthesis, RNA synthesis and protein attachment occur at different EMR wavelengths.
The shortest wavelength peak in the CCO action spectra overlaps the optical absorption of deoxygenated hemoglobin reducing the PBM magnitude of any optical stimulation.
Various conclusions can be made from these observations. Firstly, there is no ideal wavelength to optimize PBM in CCO. For that reason alone, narrow spectrum light sources (such as lasers) are not ideal for maximizing PBM response in mammalian cells and tissue. Secondly, because hemoglobin blocks red light [22], the shortest wavelength peak in CCO’s action spectra can be activated only within the epithelial layer where blood profusion is minimal.
Not all animal photobiomodulation is limited to the actions CCO and mitochondria. Other membrane bound molecules and organelles [23] such as Golgi matter, endoplasmic reticulum and others are also being investigated for PBM activity and their corresponding action spectra.
Illumination Dependence of PBM
Aside from varying by EMR wavelength, the magnitude of PBM response is condition dependent, varying with illumination which includes both the rate energy is delivered (i.e. the optical power or power density), and by the total energy delivered (i.e. the PBM dose). In biophysics, optical power (measured in Watts or W/cm2) is referred to as irradiance [24], and total energy (measured in Joules, J/cm2) is referred to as fluence [24]. In photomedicine and also in radiology, fluence is considered the “dose” of the treatment.
Like many biological phenomena, the effect of illumination on stimulatory and inhibitory effects of photobiomodulation are biphasic [25], where the amount of power and energy together determine the magnitude of PBM. Two-variable biphasic dose response of PBM can be represented by a 3D Arndt-Schultz response curve [26] shown in the following figure where energy is proportional to time at any given power.
At either low power levels (or low doses of energy) little or no PBM occurs. By increasing the power level to a substantial but safe level, the total dose can be controlled by limiting the time of exposure. At higher power levels (bright light) the exposure duration must be reduced. Conversely at lower optical power levels, the duration of exposure must be increased to produce the same degree of biomodulation.
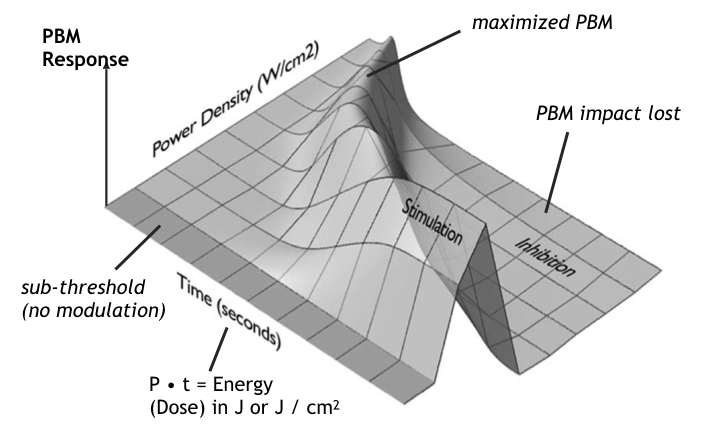
At higher doses or higher power levels PBM stimulation is diminished. At even higher levels, PBM stimulatory benefits are cancelled altogether and replaced by inhibitory effects, resulting in net negative impact of exposure on PBM activity.
Wide spectral bandwidth light sources (like the sun), although responsible for most botanical photosynthesis on earth, are inefficient at stimulating animal and mammalian photobiomodulation because the sun produces substantial amounts of ultraviolet and infrared radiation (see following figure) [27]. Typically sunlight reaching the earth’s surface comprises a mix of 53% infrared light, 42% visible light, and 4% ultraviolet light [28]. Since CCO absorption giving rise to animal PBM occurs over a narrow 300 nm wide band of the sun’s 2,250 nm overall spectrum, significant time is required to absorb significant red and infrared light from the sun. Extended exposure also includes the downside risk of sunburn (from UV overdoses) and dehydration (from over-heating by excessive infrared absorption) .
In an alternative view, if the goal of illumination is to maximize PBM within a safe temperature range [29], e.g. below 43°C to 45°C, then any heat generation not involved in producing PBM is a wasteful misuse of an organism’s PBM thermal budget, i.e. creating heat with no photochemical benefit. The problem can be avoided by limiting an illumination source to beneficial spectra, either by filtering sunlight or more pragmatically by employing artificial light sources such as lasers or arrays of LEDs as the illuminating source, and limiting the spectra to useful bands.
How Does Photobiomodulation Work ?
The action mechanism of photobiomodulation (PBM) is the transfer of light energy into molecules within cells and organelles resulting in chemical, electrochemical, & thermal reactions and transformations invoking changes in cellular metabolism and gene expression
Photobiomodulation occurs at the atomic and molecular level through a process of energy transfer [click PBM above for more information]. Photons carrying precise amounts of energy (called quanta) [30] transfer it into the molecules within living cells and their organelles. The quantity of photons (and therefore the amount of energy) absorbed by a specific cell depends on its type and structure, and on the wavelength of the impinging light.
Some portion of the light is reflected or scattered and never enters the cell. Some energy is absorbed. The remaining unabsorbed energy passes through the cell into the next layer of cells.
In accordance with the laws of thermodynamics [31], a portion of absorbed light unavoidably produces heat, i.e. producing a photo-thermal response. Other portions of absorbed light stimulates photobiomodulation in the form of photoelectric effects, photochemical reactions or some combination thereof. As shown in the following figure, an absorbed photon may cause bond breaking or bond making, resulting in a change in a molecule’s Gibbs free energy.
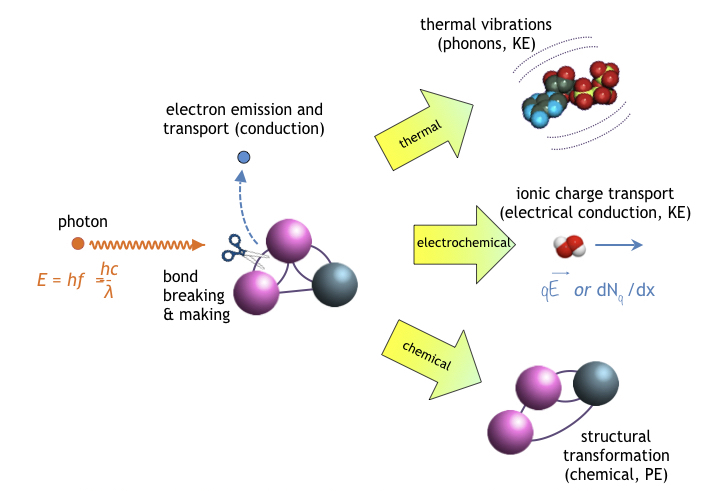
- Electron emission (and subsequent charge transport), especially in neural circuits
- Chemical transformations in molecules leading to structural changes, valance state transitions, and a corresponding change in a molecule’s stored potential energy (PE)
- Electrochemical transitions resulting mobile ions kinetically (KE) transported by force qE of an an electric field (drift conduction); or alternatively due to a charge concentration gradient (dNq(x,t)/dx in one-dimension,∇2Nq(r,t) in three-dimensions) resulting in diffusion current.
- Transient and steady-state thermally-induced molecular dynamics (thermal vibrations) comprising low-frequency oscillations (following classical Newtonian kinetics) and in some cases comprising quantized high-frequency vibrations (phonons), mechanisms observed thus far in proteins, DNA, and chromophores [32].
But what determines if light has sufficient energy to invoke PBM? According to quantum physics, a photon must exceed a specific minimum energy to stimulate a photochemical (and hence a PBM) response, below which no PBM or photochemistry occurs. So long that the energy supplied by each photon (absorbed by chromophores) exceeds the energy supplied chemically by ATP during normal metabolism, the assimilated energy should be adequate to fuel PBM.
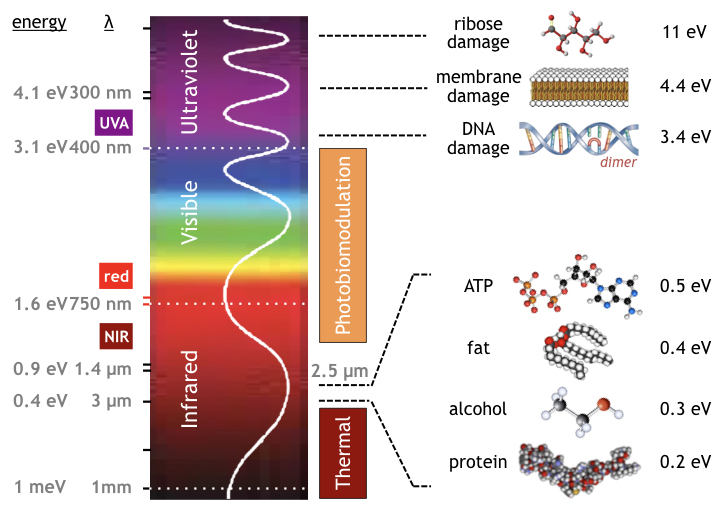
The following figure compares the energy of various wavelengths of light in the electromagnetic spectrum to the bonding energy of important biomolecules. In the hydrolysis of adenosine triphosphate [33] given by the reaction
ATP ➛ AMP + PPi + 0.47 eV
the energy molecule ATP is converted into cyclic AMP and a phosphorus molecule releasing 0.5 eV. The ATP molecule contains more energy than fat, alcohol, or protein molecules. In the electromagnetic spectrum, any IR light shorter than 2.5 µm wavelength has more energy than ATP. Unfortunately, water blocks near infrared longer than 1 µm. For this reason PBM occurs only in the NIR and visible spectrum (shorter than 1 µm) but not in the far infrared (FIR) band.
While far IR photons carry too little energy, UV For EMR in the ultraviolet spectrum, biological and physiological damage can occur including the the formation of pyrimidine dimers (DNA damage) at 3.4 eV in the UVA band [34], cell membrane damage at 4.4 eV in the UVB band [35], and ribose damage (the backbone of DNA) at 11 eV in the UVC band [36].
PBM in Mitochondrial Cytochrome-c Oxidase
Instead of relying on chloroplasts, mitochondria also contain chromophores of their own, able to capture light and either process it directly into ATP (or alternatively to enhance the conversion of sugar into ATP). One such photosensitive molecule, cytochrome-c oxidase or CCO, performs the final step of ATP generation, a process enhanced by the presence of red and near infrared light (but unlike chloroplasts not by violet, blue or orange light).
In plant cells which contain both chloroplasts and mitochondria, the biochemical interactions between the two organelles now appears to be far more complex than originally believed [38] [click on PBM tab more details]. In animal life (which lack chloroplasts), mitochondria ATP generation relies on raw materials (a source of carbon) imported into the cell from the surrounding environment.
The PBM mechanism of the cellular organelle mitochondria [39] is shown schematically in the following figure, where light in the red and near infrared spectrum is absorbed by the cytochrome-c oxidase molecule contained within the organelle, stimulating increased ATP generation and a local release of nitric-oxide (NO), a signaling molecule responsible for regulating vasodilation and circulation in animals.
The PBM process releases genetic messengers that enter the cell’s nucleus and stimulate transcription and gene expression which includes growth factors, enzymes, polymerases, and other proteins [40]. How the nucleus knows which molecules to manufacture is not known. Gene expression may be regulated by promoter molecules (the same way E-coli’s lac-operon [41] feedback and control works in knowing when to synthesize lactase and when not to) or conceivably may be controlled epigenetically, modulating regulatory molecules that silence or amplify the transcription and expression of specific genes and phenotypes [42].
During PBM, cytochrome-c oxidase also generates catalysts and reactive oxygen species (ROS) [43] including superoxide anion O2–, hydrogen peroxide H2O2, hydroxyl radical OH, and hydroperoxyl radical HO2. During PBM, mitochondria releases Ca2+ a neurotransmitter affecting cellular energetics. The generation of ATP and release of NO signal a cascade of reactions favorable to maintaining cellular vitality [For more details on the PBM of CCO, click the PBM tab above]. The results of PBM benefit the cell and the tissue, organ, and organism it comprises.
What is Photobiomodulation Therapy ?
Photobiomodulation therapy (PBT) is the therapeutic application of light energy to combat disease, repair injury, ameliorate pain, manage organ & immune system dysfunction, reduce inflammation, and address a variety of neurological and age-related health conditions.
PBT is also employed preemptively to avoid illness, prevent injury, improve brain health and cognition, promote wellness, and to enhance performance in sports & athletics.
As described previously, although photobiomodulation occurs naturally in sunlight, PBM can also be applied therapeutically using artificial light. Unlike sunlight which contains a blend of beneficial, neutral, and harmful (short-wave ultraviolet) wavelengths typically exposing large portions of the body, in therapeutic PBM, light comprising only safe and beneficial wavelengths is selectively applied locally to affected organs and tissue.
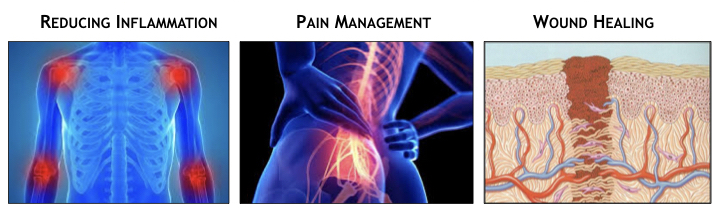
A key component of the expanding field of photomedicine, photobiomodulation therapy (PBT, PBMT) intentionally stimulates beneficial physiological effects in cells, tissue and organs. PBT treatments may comprise medical therapies treating disease, injuries, organ dysfunction, and autoimmune conditions, including inflammation, pain management, and wound healing (see figure).
Non-medical “wellness” uses include combatting everyday aches & pain, supporting fitness and good health, improving sleep & relaxation, reducing stress, improving energy, alleviating fatigue, and slowing the aging process. Other applications include boosting the immune system to preemptively ward off infectious disease.
PBT is also used in competitive sports to naturally enhance an athlete’s performance (without drugs or steroids), to reduce the risk and severity of sports injury, to manage pain from overexertion or hyperextensions, and to speed recovery when injuries do occur.
A Brief History of Photobiomodulation Therapy
As depicted in the timeline of the following figure, the first recorded uses of sunlight to promote health dates back to papyrus from dynastic Egypt [44] circa 1,550 BC. Ancient doctors noticed that sunlight and specifically certain colors (a treatment called chromotherapy) help people recover from illnesses.
Early use of light to promote health and wellness was also practiced in the Indus valley (ancient India) and in pre-imperial China. In Greece, scientists concentrated on the medical benefits of sunlight which they referred to as heliotherapy (a reference to the god Helios meaning sun). The Romans commercialized Greek light therapy into “solariums,” sun rooms that spread in popularity across Europe with the expansion of the Roman empire.
In the 1800’s, doctors and scientists began to investigate the mechanisms of phototherapeutic biomedicine. The science of phototherapy garnered international recognition in 1903 when Dr. Niels Ryberg Finsen won the Nobel Prize in medicine [45] for his use of gas-lamp and arc-lamp generated light in the successful treatment of Lupus vulgaris.
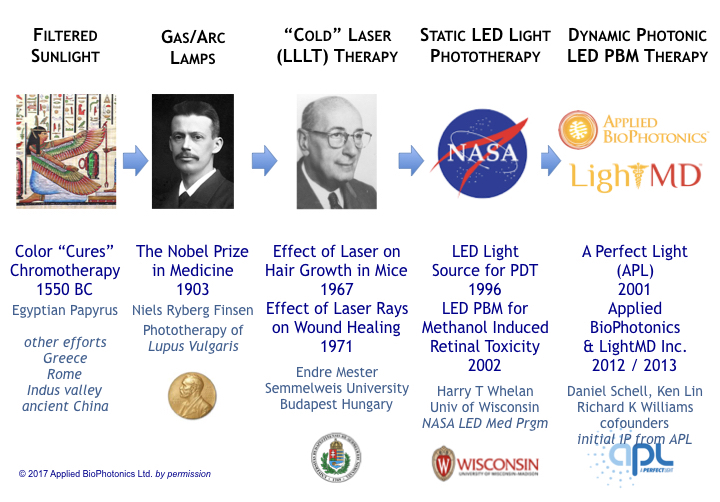
During the 1960s, the advent of laser technology brought about heath concerns that lasers (at power levels too low to cause burns) might cause cancer. Systematic studies by professor Endre Mester at Semmelweis University in Budapest Hungary revealed an unexpected result [46]. Not only did mice shaved and irradiated with laser light not contract cancer, the experimental group’s hair grew back much faster than the control group.
By 1971, studies revealed that laser light not only stimulated hair growth but accelerated wound healing. Although lasers showed exciting medical results, lasers of the 1960s and 70s were large bulky devices comprising breakable glass tubes (filled with gasses) constructed with fragile precision-aligned lenses and requiring large heavy power supplies.
In 1996, with the support of NASA’s shuttle program, Dr. Harry T Whelan of the University of Wisconsin reported the first use of LEDs as an alterative to lasers in phototherapy [47]. In 1999, he demonstrated LEDs, like lasers, effectively accelerate wound healing. In 2003 he published a seminal work on therapeutic PBM regarding methanol-induced retinal toxicity [48] – data providing evidentiary scientific support that red and infrared light stimulate ATP production in cytochrome-c, a membrane bound chromophore in mitochondria. This was a key discovery in directing research as to the photochemical, rather than a photo-thermal, origin of the true mechanism of photobiomodulation.
In general, photobiomodulation therapy efforts in the 20th century focused on static drive of lasers or LEDs – operation where light excitation was either constantly on (continuous wave operation) or pulsed at a static set of conditions throughout an entire therapy session. As a matter of record the first use of the term “photobiomodulation” occurred in 1997 [49], but was not commonly accepted at the time (in part because the term-of-art was not officially registered in the often-cited Medical Subject Headings (MeSH) data base until 2016). [50]
The turn of the millennium brought new life (and a new approach) to photobiomodulation. Starting in 2001, Dan Schell, a pioneering developer of light therapy and founder of “A Perfect Light” (APL), began experimenting with sequencing multiple wavelengths of LEDs into complex excitation patterns of varying illumination conditions and duration, and cataloging the results to define and perfect tissue-specific therapeutic regimens and protocols for disease and injury.
In 2012, Mr. Schell joined forces with Richard K Williams, electrical engineer and semiconductor physicist with expertise in molecular biology, nanotechnology and photonics. A respected serial-parallel entrepreneur, Richard was the founding CEO/CTO of the Nasdaq IPO semiconductor company Advanced Analogic Technologies Inc (ticker AATI). As an IEEE Hall-of-Fame recipient in power semiconductors & power electronics and a prolific inventor (holding over 350 patents), Richard pioneered numerous technologies including photonic systems for smartphone backlight dimming, the LED camera flash, dynamic LED backlighting (and gamma correction) for HDTVs.
Combining APL’s protocols and ABP’s biophotonics knowhow with the global manufacturing and operational expertise of Mr. Ken Lin, ABP developed and released the world’s first medical grade LED PBT biophotonic system with dynamic current control & programmable sequencing.
Featuring 3D bendable LED pads with superior optical coupling, software-based microcontroller waveform synthesis, hierarchically based menu and intuitive UI/UX (for unambiguous user selection of tissue specific protocols), ABP’s PBT system conferred unparalleled therapeutic control to physicians and clinicians, making it the world’s most flexible and versatile PBT system.
Of particular note, ABP’s G1 photobiomodulation therapy system was the first menu-driven software-based PBT system in history to undergo and pass an exhaustive US FDA audit as well as earning industry-recognized GMP certification by Taiwan’s rigorous FDA. ABP has also developed an extensive IP portfolio of US and international patents in photobiomodulation. Its go-to-market strategy included bifurcating its PBT business into two companies, one for medical products, the other for medical services and client training, vis-à-vis…
- Applied BioPhotonics Ltd designs & manufactures medical-grade professional PBT systems, obtaining all necessary governmental and industry safety certifications and approvals.
- LightMD Inc investigates therapeutic strategies and documenting case studies while working with and providing medical advise & support to doctors and hospitals using ABP products.
PBT – Therapeutic Applications of PBM
The therapeutic application of photobiomodulation is referred to photobiomodulation therapy (aka PBT or sometimes PBMT). Generally described in the context of treating humans and other mammals (e.g. dogs, cats, horses, and camels), PBT addresses an amazingly wide range of physiological conditions, primarily because PBM naturally occurs in nearly all tissue types, i.e.
- Nerve tissue
- Muscle tissue
- Epithelial tissue
- Connective tissue
The efficacy of photomedicine in general and PBT in particular, depends on the nature of a patient’s condition, the treatment regimen performed, and photonic delivery system employed.
With over 300,000 papers published in PubMed alone, the preponderance of empirical evidence supporting the efficacious application of PBM regimens is staggering. Used both as primary care or adjunctively, PBT is no longer limited to the purview of alternative medicine. Its ability to successfully treat disease and injury rivals today’s best pharmacological solutions. Because, however, of the diversity of conditions treated (including chronic, rare, and fatal diseases), it is difficult to define optimum therapeutic regimens simply based on a review of published papers.
Patients often turn to PBT as an alternative to complex invasive surgical procedures (such as joint replacement) or dangerous / toxic pharmacological regimens, especially those for which a doctor is unable to confidently offer therapeutic success. For example, in veterinarian medicine, dogs with partial paralysis frequently regain full motor control with PBT avoiding the need for risky, back surgery (costing over $10,000 USD). In other cases, when drugs are inefficacious or contraindicated (e.g. in numerous chronic diseases unresponsive to medication or in immuno-compromised patients) PBT is the oftentimes the only remedy available. And unlike drugs, PBT is non-addictive and non-toxic, with no adverse impact on liver, kidney, and immune function.
PBT’s ability to combat a wide range of seemingly unrelated medical conditions is based on its fundamental mechanisms of action – delivering photons as uncharged energy to cells and organelles in order to enhance a cell’s metabolism and intrinsic (natural) repair mechanisms through photochemical processes. Using uncharged particles is preferable over electric and magnetic therapies to avoid disturbing the body’s natural electrochemical reactions. PBT’s use of physical mechanisms to stimulate chemical reactions is in sharp contrast to the use of pharmaceuticals, which relies solely on chemical reactions oftentimes involving molecular agents (compounds foreign to cells and tissues) with unforeseen consequences and side effects.
PBT’s ability to successfully treat seemingly unrelated tissue types is based on the commonalities of living cells (not their differences), whereby in mammalian life for example:
- All differentiated cell (and tissue) types come from a common stem cell ancestry.
- All cells require energy to function.
- All cells (except certain blood cells) contain mitochondria.
- All cells contain genes encoded in DNA and converted into proteins using RNA transcription.
- All cells regulate gene expression to dynamically control their phenotype in response to a changing environment.
- All cells are influenced by tissue pH and locally by blood perfusion and their oxygen supply.
- All cells must communicate with other cells (electrically, chemically, or electrochemically).
- All cells include cellular repair mechanisms (including DNA repair via endonuclease activity).
- Most cells contain light sensitive chromophores affecting metabolic processes.
Despite invoking common mechanisms-of-action in all animal cells, the beneficial effects of PBT are tissue specific varying for nerve, muscle, epithelial, and connective tissue type in accordance with their tissue type. As such photochemical mechanisms present in all cells produce different type of responses based on the illuminated tissue type. Although illumination conditions of a PBT treatment, i.e. light wavelength, irradiance, fluence, modulation, optical transmission and absorption, determine PBT’s energy coupling and magnitude of PBM in specific tissues, the type of cytological response is determined by the cell type, not the physics of illumination.
Based on published therapeutic accounts, clinical trials, and ABP’s own case studies, the following figures describe conditions amenable to treatment with photobiomodulation therapy as arranged by tissue type, namely nerve, muscle, epithelium, and connective tissue.
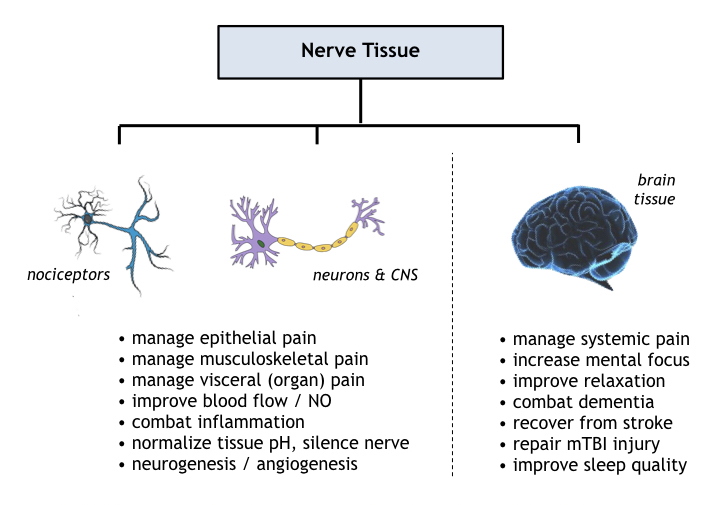
Nerve Tissue
Reported uses of photobiomodulation therapy on nerve tissue comprise the brain & central nervous system as well as the peripheral nervous system including afferent and efferent nerves for skeletal muscles, for visceral organs, and for skin. Primary PBM mechanisms in nerve tissue comprise improved circulation, reduced tissue inflammation, increased oxygen supply, normalizing tissue pH, accelerated wound healing, and invoking selective neurogenesis.
PBT benefits to clocked neural circuitry, i.e. brain and autonomic nervous system, includes regulation of peristaltic contractions (digestion, breathing, cramping) and brainwave synchronization (evoking relaxation or mental focus). Investigatory brain therapies include treatments for mTBI/concussion, attention deficit disorder (ADD), and Alzheimer’s/dementia.
One major neural application of PBT is in pain management– non-pharmacological analgesia not subject to the adverse side effects and kidney damage of steroidal & NSAID compounds or at risk for the addictive dangers of opiates and opioids. Mechanistically, nociceptors (pain sensors) in the skin, organs, connective tissue, and on bone surfaces respond directly to PBT, ameliorating pain locally and reducing pain signal transduction to the brain. PBT provides relief for all nociceptors including mechanical, acidic, traumatic cold/hot, and multifunction types.
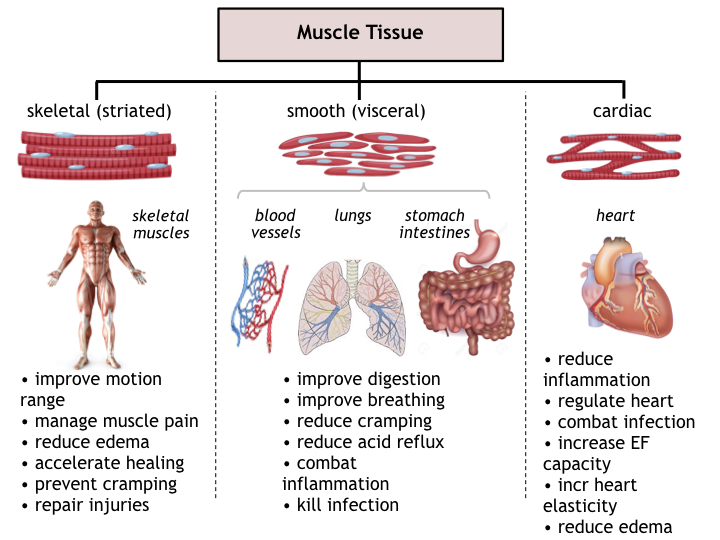
Muscle Tissue
Reported uses of photobiomodulation therapy on muscle tissue comprise effects on skeletal (striated) muscles, visceral organs via smooth muscles, and cardiac muscles. General effects of PBT on muscle tissue involve improved circulation and tissue oxygenation as well as combatting inflammation and supporting immune response to fight microbial infections, and to accelerate in tissue regrowth in wounded muscles.
Specifically in skeletal muscles, benefits of PBT treatments include increased tissue oxygenation and enhanced biokinetic capability, an increase in the lactic-acid threshold of cramping, and management of local inflammation and edema. PBT generated increases in elastin and collagen also improve muscle flexibility and an expanded range-of-motion, thereby minimizing risks of hypertension, sprains, and muscle tears. In athletics and sports, treatments may be used prior to strenuous activity to minimize the risk of injury (and legally enhance performance), as part of an exercise regimen to keep muscles warm and loose between events, to enhance breathing (lung capacity and blood oxygen), or after activity to gently relax muscles, inhibit cramping, and enhance stretching.
The benefit to visceral organ (smooth) muscles and to cardiac muscle tissue is primarily to enhance blood perfusion, deliver oxygen, and normalize pH to combat inflammation and infection risks. PBT also reduces swelling, edema, and pain not only in skeletal muscles, but in autonomic muscles of the diaphragm (lungs), stomach, and intestines.
Epithelium Tissue
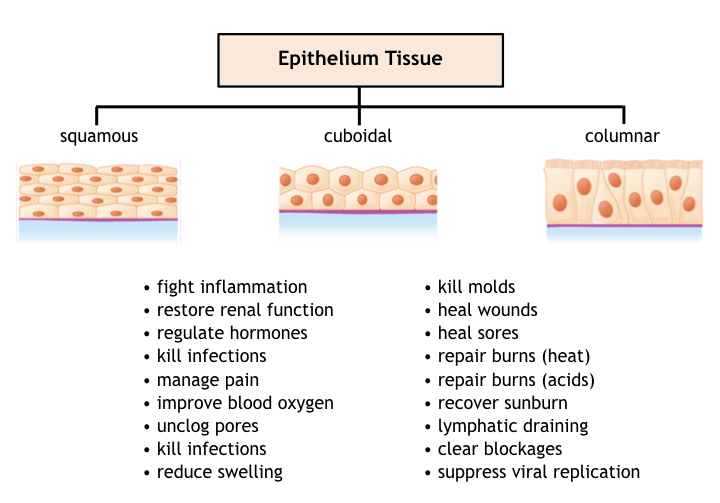
Epithelium tissue is present throughout the body both as skin (the body’s protective layer for resisting abrasion and environmental damage), and for lining internal (visceral) organs in the digestive, respiratory, endocrine, and immune systems, conferring not only protective benefits but also for realizing semi-porous membranes used in passing hormones, enzymes, mucus, digestive products, and other biochemical molecules into or out of organs.
Photobiomodulation therapy has been demonstrated on epithelium tissues including simple and stratified squamous, cuboidal, columnar, and transitive cell types present in a variety of organs.
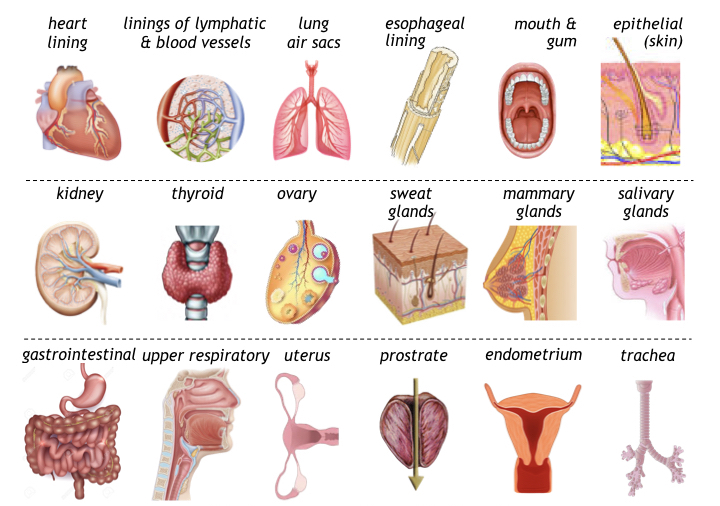
General effects of PBT on epithelium tissue involve preserving tissue integrity by rebuilding elastin and collagen, preventing infection and inflammation (particularly surrounding wounds or ulcers), maintaining a healthy pH, and repairing injuries. In hormonal glands such as the thyroid, prostate, ovary, pituitary and thyroid glands, PBT helps regulate hormonal production and control metabolism. In secretory glands such as salivary, mammary, and sweat glands, PBT can also be used to unclog pores and prevent infection and inflammation.
The anti-inflammatory benefits of PBT also assists epithelium tissue lining vesicles of the circulatory and lymph system, and in bronchial air sacs to maintain healthy cardiopulmonary function. This mechanism beneficially supports oxygen delivery by blood perfusion (even though most epithelium lacks blood vesicles), instead receiving nutrients from adjacent tissue.
Connective Tissue
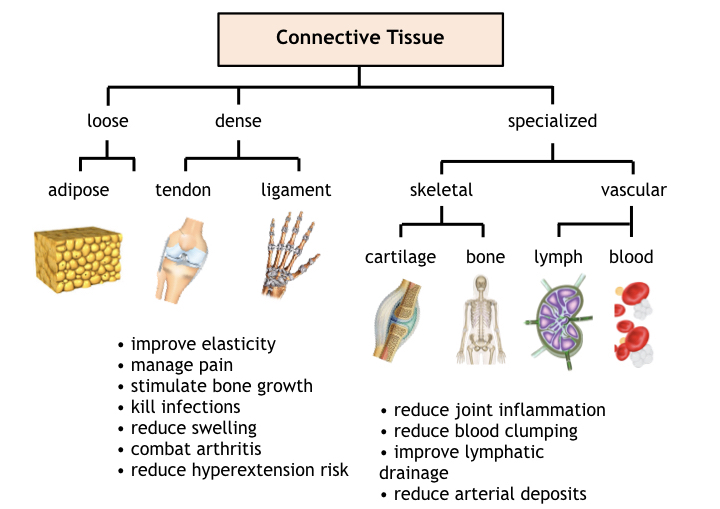
Connective tissue present throughout the body comprises loose connective tissue in adipose, dense connective tissue (in ligaments and tendons); specialized skeletal connective tissue in cartilage and bone; and specialized vascular connective tissue comprising blood and lymph tissue.
Considering its diverse functions, PBT affects connective tissue in a variety in accordance with the functional or structural role it serves in the body. In adipose tissue, PBT reportedly shrinks adipose cells through the release of triglycerides and numerous other mechanisms still under investigation. PBT is also known to increase proliferation of adipose derived stem cells for regenerative medicine including in vivo and in vitro cartilage therapy regimens.
For dense connective tissue comprising ligaments and tendons, PBT is generally used to manage joint pain, treat hyperextensions, and repair other sports injuries. Healing and pain management of connective tissue involves several mechanisms including improving circulation, stimulating tissue repair, reducing swelling, and combatting inflammation. For special skeletal connective tissue, PBT enhances the post trauma growth rate for bone and cartilage.
Benefits of PBT in the circulatory system include improved blood perfusion, reduced clumping of erythrocytes, enhanced immune response, reduced infection rates, maintaining a healthy population of lymphocytes and T-cells, and shorter recovery times from viral attacks. PBT also helps manage the proper immune response to avoid sensitization and autoimmune distress.
Biophotonic Control of PBT Treatments
In modern photobiomodulation therapy, an array of LEDs or a low power laser is applied locally to tissue or organs therapeutically to stimulate the body’s natural healing processes.
In all forms of photomedicine including PBT, the optical energy delivery system comprises an optical source (i.e. LEDs or lasers), a source of power (a regulated power supply), an electronic control system, and a user interface (UI/UX). Such systems are referred to collectively as biophotonics (where the prefix bio refers to “living cells” and photonics means the electronic control of photons, i.e. light). Requirements of a biophotonic PBT system include:
Delivering sufficient energy to achieve an efficacious therapeutic effect via PBM.
Preventing energy overdosing to avoid inhibition of therapeutic benefit (biphasic response).
Deliver sufficient optical power to execute treatments in a reasonable time, comfortable for both a patient and for a therapist administering a treatment.
Control optical delivery to prevent eye damage and manage burn risk (safety)
Cover a sufficiently large treatment area to practically treat an organs or muscles without undue repetitions or extensive treatment times.
Minimize energy loss from optical reflections and refractive scattering.
Examples of practical consideration in biophotonic design are shown in the following figure:
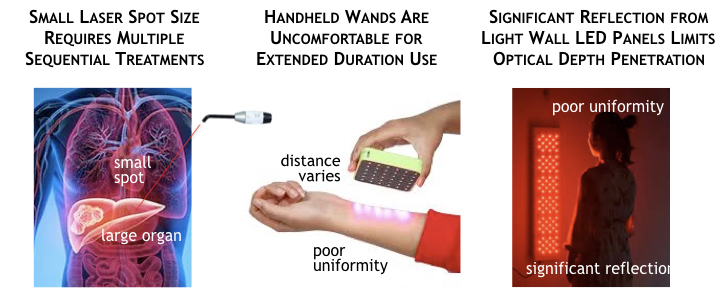
Practical considerations of a photonic system for PBT include optical treatment area; therapist and patient comfort; optical coupling efficiency and light uniformity; and safety. In regards to optical areal coverage, using a small spot size light (lasers or single LEDs) to treat large organs or muscles requires repeatedly moving the source and performing the same treatment over again. This arduous “treat, move, repeat” process can be uncomfortable for the therapist and time consuming for the patient, especially if the time needed is over several minutes per location.
Another important aspect of a biophotonic system is light uniformity. Despite the disadvantages of naturally small optical source area, lasers beneficially exhibit an optical power density does not depend on the distance between the laser’s beam source and the patient’s skin.
The same is not true for LEDs where light intensity penetrating the skin and the depth of the penetrating light both are affected by the distance between the LED and the patient. The use of a handheld or LED wall panel shown previously clearly illustrate significant reflection and poor light uniformity of the patient’s skin. Reflected or refracted light is wasted energy that does not contribute to photobiomodulation or therapeutic effects. Poor light uniformity means that’s some tissue may receive too much light while other portions remain almost in the dark.
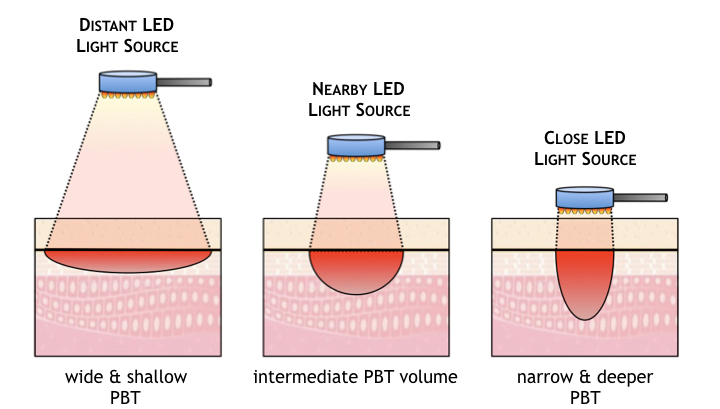
Another key aspect related to the light source is optical penetration depth. As shown below, for any given wavelength of light, the penetration depth of absorbed light from an LED source depends on the distance between the LED or LED array (shown here as a handheld wand) and the patient’s skin. If the distance is great the light energy is spread across a larger tissue area but penetrates less deeply. If the distance is small, the optical power density on the skin’s surface is higher and the light penetrates more deeply, but the treatment area is reduced.
During a treatment of minutes or tens-of-minutes, holding a wand in place at a consistent distance and in one specific location is impractical at best and uncomfortable for a therapist. The therapeutic benefits of PBT vary wildly of the optical power delivery is unpredictable [see ABP ‘s Product section for a solution to this uniformity & depth dilemma].
The most critical consideration in the design of a medical biophotonic system is one of safety – to precisely control the magnitude and duration of energy delivered to a patient’s tissue to maximize efficacy while avoiding levels of light dangerous to skin and to eyes.
As shown previously, although holding a wand in place for extended times may be uncomfortable or impractical and produce inconsistent results, any attempts to accelerate a treatment by increasing optical power commensurately increases the risk of accidentally inflicting burns or causing blindness.
As illustrated in the following figure, the power of a light source (shown on the y-axis) is the key parameter setting the function and safety of a biophotonic system. At high power levels, cells are burnt and destroyed by light, i.e. ablated. Short duration high-power laser pulses are employed in laser surgery [38]. In intense pulse light (IPL) therapy (a somewhat bizarre method for stimulating skin healing by first damaging it), high power flash bulbs are used to burn off epithelial layers [39].
The threshold of eye safety (see eye icon) occurs at a much lower power level than the onset of cell ablation. For power levels above this threshold, proper eye safety must be ensured using safety glasses. Safety glasses are critical requirement in laser photomedicine in part because laser pulses can occur so quickly the eyes have no time to blink to stop a dangerous exposure.
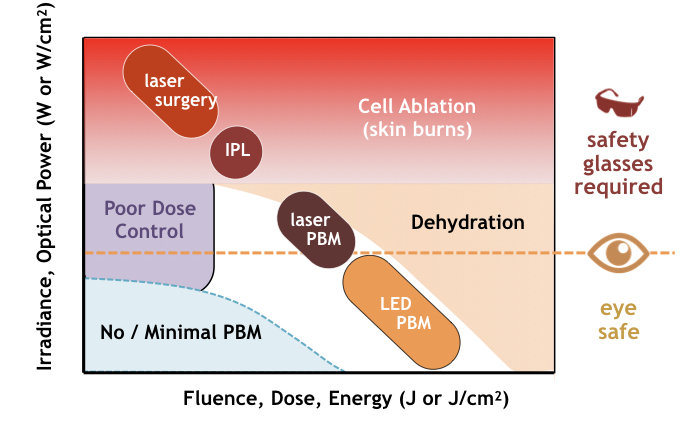
In the same graph, the x-axis represent the total energy (dose) delivered in a treatment. Dose, measured in joules (or joules per area), is the product of power and time. For constant power treatments E = P•t, dose is essentially a measure of time.
At high power levels used in laser surgery, typically 10 MW/cm2 to 10 GW/cm2, pulse durations are kept short, using durations of microseconds to picoseconds to minimize heat diffusion into surrounding tissue. In sub-picosecond laser surgery cells are literally vaporized with virtually no heat transfer to neighboring cells. In contrast, intense pulse light (IPL) is intentionally a thermal process lasting 1 to 10 ms (that’s why it stings) with irradiances of 10 kW/cm2 and total fluences in the 10 to 50 J/cm2..
Photobiomodulation treatments are administered at much lower power levels than ablative processes (typically under 1 W/cm2). Reported values of irradiance vary widely based on treatment area, especially for lasers where the spot size depends on light delivery methods (direct, fiber, optics). Given the biphasic behavior of photobiomodulation, PBT doses (reported in journals typically ranging from 1 to 10 J/cm2) are selected to exceed a minimum PBM threshold yet below the onset of tissue dehydration.
In order to minimize therapy times to accommodate hand held probes, laser PBT employ higher power levels than LED PBT, conditions not well suited for low dose therapies.
Since lasers can operate either above or below the optical safety limit, even their clinical use at low power levels (cold lasers) requires constant supervision. The religious use of safety glasses throughout a treatment session is mandatory because a laser malfunction, however unlikely, could cause a rapid unsafe power surge. In contrast, PBM using LED-based delivery systems are intrinsically eye safe, incapable of causing eye damage.
Moreover, aside from its higher safety risks of a malfunction, there is no therapeutic benefit to operate a laser at high levels of irradiance just to more quickly deliver small fluence (low-dose) PBT treatments. To the contrary, at high optical power densities, slight variations in laser beam uniformity and in treatment time (i.e., the time a treatment is performed on a specific location) may cause a large change in dose and more variability in treatment efficacy.
One common misconception (or misrepresentation) in laser therapy is that more powerful lasers send light deeper than lower power sources. This assertion is simply not supported by science. Higher irradiance (optical power) simply means more photons are delivered at one time (a brighter light). The energy an individual photon has (or how deeply it can penetrate into matter) has nothing to do with the brightness of light or the speed the photon travels (which is in fact constant in a given material). Instead, according to modern physics (quantum mechanics) the energy of a photon (and therefore its corresponding penetration depth) is determined solely by its wavelength (color).
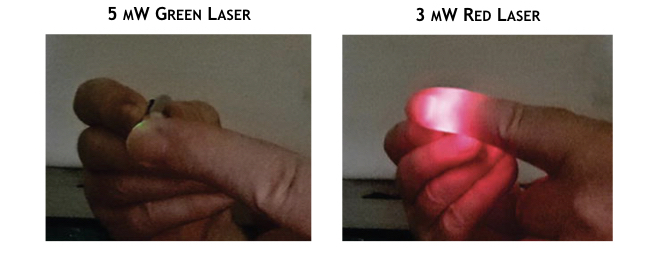
Accordingly, the penetration depth of light in photobiomodulation therapy depends on its wavelength and the tissue being treated. As shown in the following figure, for example, light from two lasers having different colors penetrate the finger differently. Although a significant portion of red light from a 3 mW laser passes entirely through the finger, the shorter wavelength green light from a more powerful 5 mW is totally blocked, further reinforcing the fact that depth of a PBT treatment is not determined by the power of the source.
Topics in Photomedicine
Photomedicine and biophotonics have grown into major topic of research and journal publications today, using a variety of names including laser therapy, cold lasers, LED therapy, and phototherapy and LLLT, a confusingly ambiguous term used to mean both low-level laser therapy and low-level light therapy. More recently it was decided that the best term for non-thermal photochemical processes should be referred to as photobiomodulation (PBM) and the therapeutic use of PBM as photobiomodulation therapy (either PBT or PBMT). By this definition PBM and PBT includes PBM therapy, LLLT, phototherapy, LED and laser therapy, and cold lasers.
